ABSTRACT
This article deals with the use of effect-based methods for the qualitative assessment of the state of surface waters in the context of Directive 2000/60/EC establishing the framework for Community activity in the field of water policy and the upcoming amendment to Directive 2008/105/EC on environmental quality standards. The implemented monitoring of priority substances and specific pollutants is not able to capture all sources of pollution that negatively affect surface water quality. Likewise, current practice does not allow a comprehensive assessment of mixtures, including emergent pollutants, metabolites, and transformation products of substances on water quality. Effect-based methods are a suitable tool for ecotoxicological evaluation of pollution, which considers all substances contained in the sample and possible effects of mixtures (synergistic effects). They thus provide important additional information for the results of the assessment of the state of surface water bodies.
INTRODUCTION
The Water Framework Directive 2000/60/EC requires the Member States of the European Union (EU) to adopt an integrated approach to the monitoring and assessment of surface water quality. In the Czech Republic, monitoring takes place in accordance with the requirements of Decree 98/2011 [1] as amended by Section 21 of the Water Act [2] and environmental quality standards throughout the EU. However, for technical and economic reasons, the current monitoring of the chemical state of water does not allow analysis, detection, and quantification of all substances present in the aquatic environment [3, 4]. It is primarily focused on monitoring selected regulated chemical substances – priority substances and other pollutants which are known to pose a danger to the aquatic environment. However, this information does not tell us anything about their actual cumulative effects in the aquatic environment. In addition, it is necessary to include the action of so-called emergent micropollutants in the monitoring. These include medicines and cosmetics, biocides, polar pesticides, endocrine disruptors, their metabolites, and transformation products. In addition, substances present in the aquatic environment create mixtures whose final effect cannot be predicted on the basis of chemical analysis alone.
The main goal of the project “Using effect-based methods to assess the status of surface waters in the context of the Water Framework Directive” is to create a methodology for the assessment of water pollution using effect-based methods (EBM), the use of which would be appropriate to include in the Water Framework Directive 2000/60/EC (WFD) [5]. This type of monitoring is a useful ecotoxicological tool for the assessment of water pollution, serving as a screening method, allowing to precisely target other types of monitoring and, subsequently, find the origin of the pollution and set measures to improve water status. This issue is dealt with in detail, for example, by US EPA documents [6–8]. Simultaneously, an amendment to Directive 2008/105/EC is currently being discussed at the Member States level, which in Article 8a introduces a new obligation to monitor the presence of estrogenic substances in water bodies using EBM for a period of two years; in case of positive findings, the following hormones will be monitored by conventional analytical methods: 7-beta-estradiol (E2), estrone (E1), and alpha-ethinyl-estradiol (EE2).
EBMs are analytical methods using whole organism responses (in vivo) or cellular responses (in vitro) to detect and quantify the effects of different groups of chemicals and to determine relevant toxicological endpoints [4]. Incorporation of these methods into current monitoring would therefore allow evaluation of the effects of complex pollutant mixtures occurring in the environment according to the mechanism of their action [5, 7–9].
EBM can help prioritize problematic groups of substances; this can be used for designing targeted measures to reduce their introduction and to improve water quality. Another important aspect of their use is the potential to reduce the burden associated with monitoring an ever-growing list of priority substances and pollutants. EBMs are suitable for linking the monitoring of the chemical and ecological status of the aquatic environment and can help determine the causes of the unsatisfactory ecological water status, as well as to identify other substances that may be a threat to both aquatic ecosystems and human health. Using EBM enables a cost-effective risk analysis where the absence of an effect implies the absence of toxicological risk.
The methods used were chosen with a view to covering different mechanisms of action of toxic substances based on the required sensitivity. The basic document was the EU technical report [11] and other sources [10, 12]. Considering the small volume of the sample after the necessary pretreatment, ecotoxicity tests were chosen on representatives of two trophic layers of aquatic ecosystems – decomposers (bacteria Aliivibrio fischeri), and primary producers (green algae Raphidocelis subcapitata). Mutagenicity was determined using the Ames fluctuation test [12, 14, 15]. In recent years, water quality testing has been focused on estrogens and substances with an estrogenic effect. The YES test (yeast estrogen screen) [16, 17] was used to determine the level of estrogens and estrogenic substances recommended for monitoring at the EU level [12].
METHODOLOGY
Selection of profiles and sampling plan
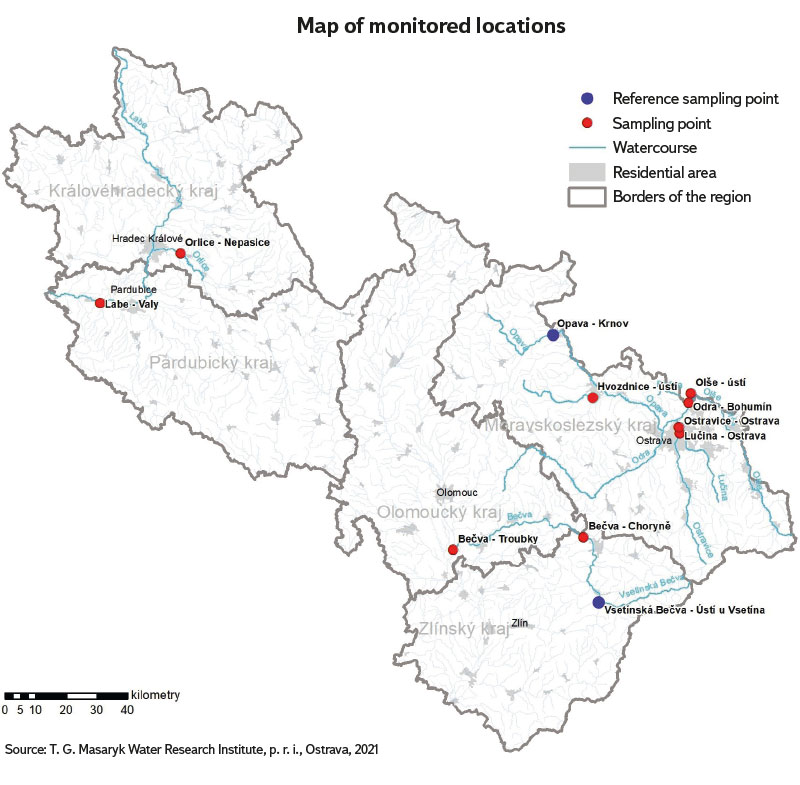
Eleven locations in three river basins (Odra, Morava, and Labe basins) were selected for the assessment of surface water status using EBM, based on CHMI data and consultations with the Povodí state enterprises. Nine locations show long-term poor water quality, which in most cases is caused by exceeding limit of phosphorus and nitrogen concentrations, but also by an increased occurrence of some priority substances and other pollutants and metals (according to Government Regulation No. 401/2015 Coll. [17]). Two locations were selected as the reference ones with reported long-term good water quality. The selected profiles are listed in Tab. 1; the site map is shown in Fig. 1.
Tab. 1. Water profiles overview
Fig. 1. Map of monitored locations
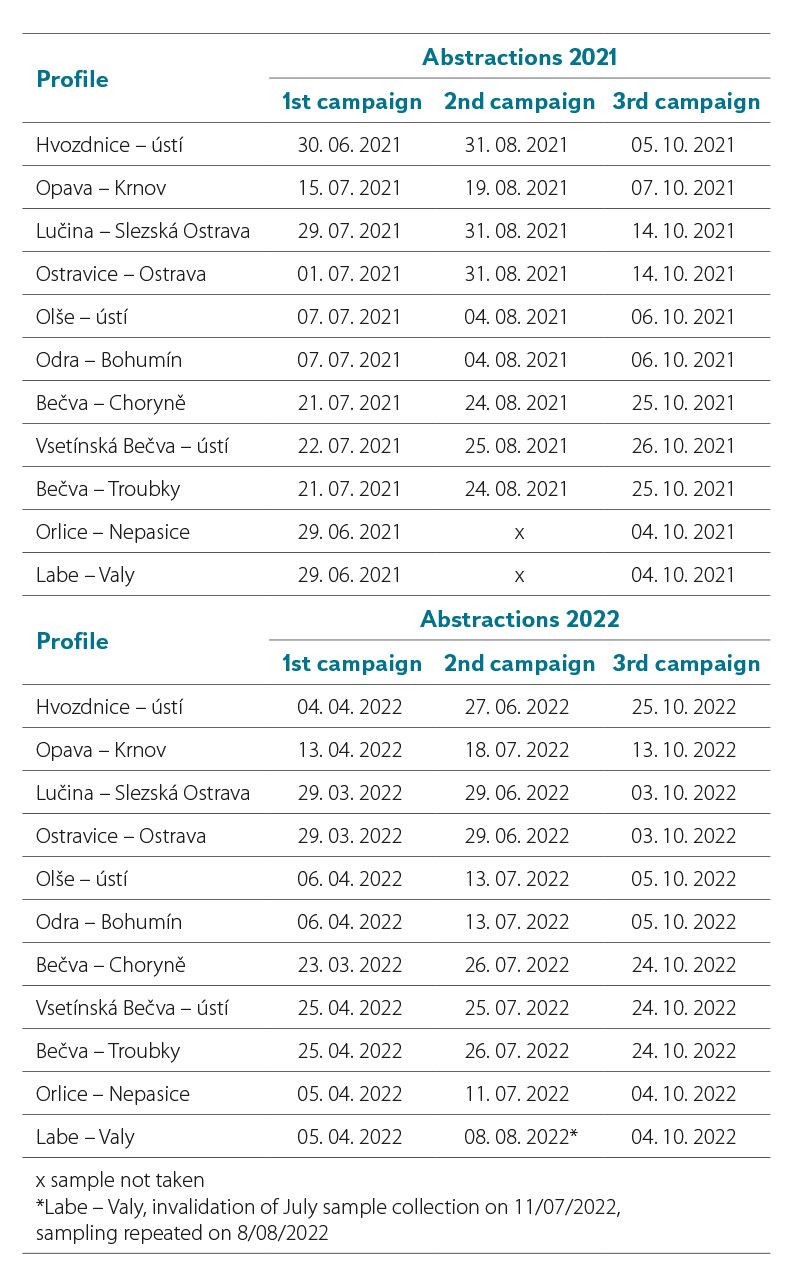
A total of six sampling campaigns took place in 2021 and 2022 (Tab. 2).
Tab. 2. Overview of abstraction dates in selected monitored profiles
METHOD USED
Pretreatment of samples
Pretreatment of samples consists of concentrating the pollution contained in them. This procedure is chosen on the basis of the assumption that an increase in the concentration of active substances will make it possible to model their possible chronic effect within a significantly shorter exposure time, i.e., using acute toxicity tests (the effect is influenced by the indirect dependence of the concentration on exposure time).
Concentration of the collected samples was carried out according to TNV 75 7231 [18]. XAD resins were added to 20 litres of surface water sample and the sample was mixed for 24 hours. The adsorbed substances were then washed kilometres with solvent and transferred to an aqueous 1000x concentrated sample. It was subsequently used for evaluation using selected effect-based methods:
- Toxicity test with decomposers: Microtox test with the luminescent bacterium Aliivibrio fisheri according to ČSN EN ISO 11348 standard [15].
- Toxicity test with producers: miniaturized green algae growth inhibition test according to ČSN EN ISO 8692 standard [16].
- Endocrine disruption – evaluation of estrogenicity using a commercial kit using the yeast Saccharomyces cerevisiae (S-YESMD New Diagnostic).
- Genotoxicity – determination of direct mutagenicity using the Ames test with a bacterial culture of Salmonella typhimurium (strains TA 98 and TA 100) according to ISO 11350 : 2012 standard [21].
When performing tests, increased toxicity of blank samples was noted in some cases. Since the influence of the solvent was ruled out, we assumed the influence of residual toxicity after conditioning of the polymer resins (XAD resins). XAD resins are commercially supplied and stored wet in containers with added sodium chloride and sodium carbonate to prevent unwanted microbial growth. In addition, other undesirable substances can be adsorbed onto the resins from the production process, which can negatively affect the results of the ecotoxicity tests themselves. The original procedure of cleaning with methanol and subsequent rinsing with demineralized water was adjusted based on the data obtained from the research. The resins were cleaned and conditioned in Soxhlet extractors for 8 hours with methanol, followed by 8 hours with acetone [22]. These are solvents that are used in the subsequent steps of testing even during the extraction of sorbed substances and are also recommended by manufacturers of XAD resins (e.g. Supelco, Sigma Aldrich).
In addition to the change in the conditioning of the XAD resins, there was also a change compared to TNV 75 7231 in the extraction method. The mentioned standard recommends acetone for extraction. Methanol was added as a second reagent, which is a more polar solvent compared to acetone and is suitable, for example, for the extraction of a wide range of pesticides and pharmaceuticals, where the extracted substances should be expanded by the mentioned substances with a higher polarity. Changes to the extraction procedures included mixing the resins with the sorbates in the column with methanol for 30 minutes. The methanol extract was poured from the columns into prepared containers. Acetone was then gradually added to the resins in the columns, in two subsequent steps, for a period of 2× 15 minutes. The first acetone extract also contained a small amount of methanol from the first extraction step; therefore, after 15 minutes the sorbed substances on the resins were extracted again with pure acetone. The final acetone extract was created by combining the two acetone extracts.
The resulting methanol and acetone extracts were first concentrated to a volume of 5 ml on a Heidolph vacuum rotary evaporator (water bath temperature of 50 °C, pressure of 300 mbar for the methanol extract and 550 mbar for the acetone extract) [22–25]. The extracts were extended with nitrogen to a final volume of 100 µl. The concentrated acetone and methanol extracts were filled with demineralized water to a volume of 10 ml. In the last step, these samples were mixed together and a 1,000× concentrated aqueous sample with a volume of 20 ml was created for effect-based analyses.
Determination of estrogens
Determination of selected estrogens (E2 – 17β-estradiol, EE2 – 17α ethinyl estradiol, E1 – estrone) by LC/MS method was carried out on 15 selected samples of concentrated surface water from 2021 on a liquid chromatograph in the laboratories of the Department of Hydrochemistry, TGM WRI in Prague.
Toxicity test – luminescence test with A. fischeri
Determination of the inhibitory effect of the samples on the light emission of the marine luminescent bacteria Aliivibrio fischeri was carried out according to ČSN EN ISO 11348-2 standard. These are marine aerobic, heterotrophic, gram-negative bacteria capable of bioluminescence. Light emission is produced by the catalytic effects of the enzyme luciferase on the low molecular weight substrate luciferin. Due to the toxic substances contained in the tested sample, the luminescence emitted by these bacteria decreases. This reduced value is recorded and compared to the control test (Figs. 2 and 3). The results of the analyses of the bioluminescence test are shown in EC50 values (ml/l) in Tab. 2. These values represent the concentration at which there was a 50 % decrease in luminescence compared to the control test.
Fig. 2. Example of luminescent bacteria A. fischeri cultured on a Petri dish

Fig. 3. Measurement of luminescence intensity changes during the test
Toxicity test – miniaturized algae test with R. subcapitata
The freshwater green algae growth inhibition test is based on ČSN EN ISO 8692 standard (Fig. 4). Due to the limited number of concentrated samples obtained, a miniaturized algae test method was used. The miniaturized version does not take place in Erlenmeyer flasks, but in 96-well microtiter plates. The basic solutions and test conditions remain identical to the already mentioned standard. The essence of the test consists of cultivating the algal culture R. subcapitata in samples with the addition of a nutrient medium necessary for the growth of algae. Cultivation takes place in a test room with a constant temperature of 22 °C, with a light intensity of over 6,000 lx. After 72 h, the specific growth rate of the examined samples is compared with the control sample. The resulting values of the analysed samples are expressed in % of inhibition (or stimulation) of algae growth compared to the control sample.

;
Fig. 4. Example of miniaturized algal test (left); microalgae R. subcapitata (right); standard version of the algal test running in Erlenmeyer flasks (bottom)
Genotoxicity test – Ames fluctuation test
The Ames fluctuation test (ISO 11350) was used to detect the presence of substances with a mutagenic effect. Using two genetically modified bacterial strains of Salmonella enterica subsp. enterica serotype Typhimurium TA 98 and TA 100, this test monitors the occurrence of direct and indirect mutagens in the aquatic environment. By using both mentioned strains (TA 98 and TA 100), it is possible to detect substances in the samples that induce point mutations (base substitutions and displacement mutations) in genes encoding enzymes that participate in the biosynthesis of the amino acid histidine. A two-fold increase in the number of revertants is considered significant (Fig. 5).
In the Ames test, evaluation of the cytotoxic effects of the samples on Salmonella bacterial strains is also recommended, based on the evaluation of the growth rate of bacterial strains compared to control samples. Detection of cytotoxicity is recommended by ISO 11350 standard only for the Salmonella strain TA 98. With the strain TA 100, the results may be distorted due to lower growth rate. Due to significant staining of some analysed samples which distorted the cytotoxicity evaluation, it will be necessary to optimize the evaluation.
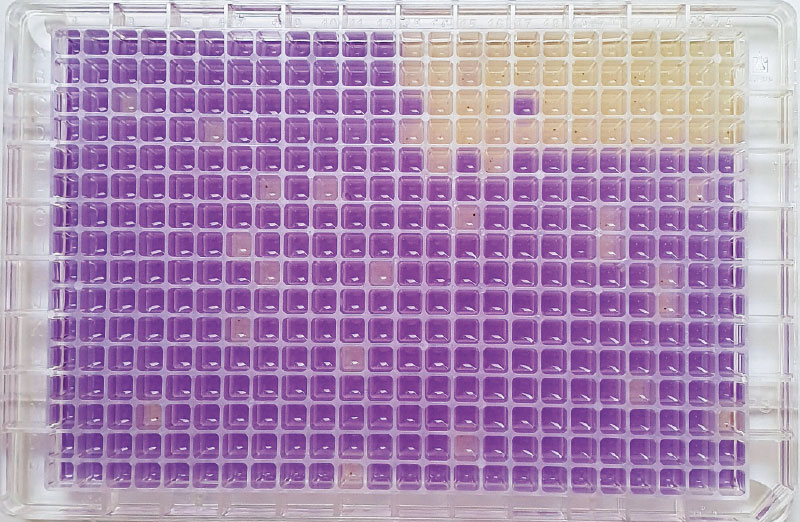
Fig. 5. Ames fluctuation test on a microtitre plate
Ames test with metabolic activation (S9+)
In 2022, samples were also tested in a variant of the Ames test with metabolic activation S9+, monitoring indirect mutagens which require metabolic activation by liver enzymes in order to manifest their mutagenic effect. In this variant, samples with a concentration of 500 ml/l were tested.
Test to determine estrogen potential – Yeast estrogen test (YES test)
The YES test method is aimed at determining the estrogenic potential of aqueous samples. The procedure is based on ISO 19040-1 : 2018 standard [26]. This colorimetric YES test uses recombinant Saccharomyces cerevisiae yeast cells genetically engineered to express the human estrogen receptor alpha (hER). Depending on the presence of estrogenic substances in the sample, the colour of the indicator (CPRG) changes as the substance binds to the estrogen receptor. This initiates the expression of the reporter gene and the synthesis
of β-galactosidase, which is released by the cell into the medium, in which it catalyses the conversion of the yellow CPRG substrate to red (Fig. 6). The intensity of the red colour is related to the level of estrogenic activity of the sample and is evaluated spectrophotometrically at OD570 nm. The measured optical density (OD) is directly correlated with the amount of β-galactosidase released, and thus with the activity of the test substance that has bound to the receptor.
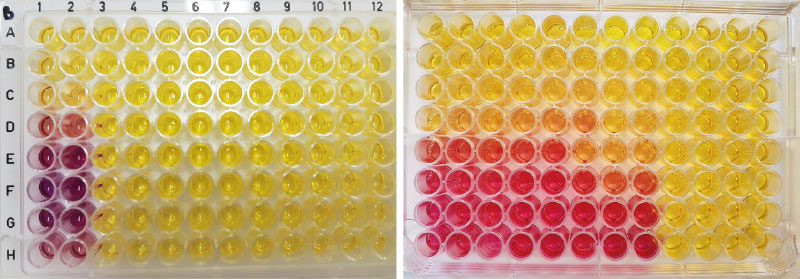
Fig. 6. YES test
RESULTS
Determination of estrogens
Measurements were carried out on samples collected during three campaigns in 2021 in selected profiles. None of the estrogens could be determined in the samples as their values were below the detection limit.
Toxicity test – luminescence test with A. fischeri
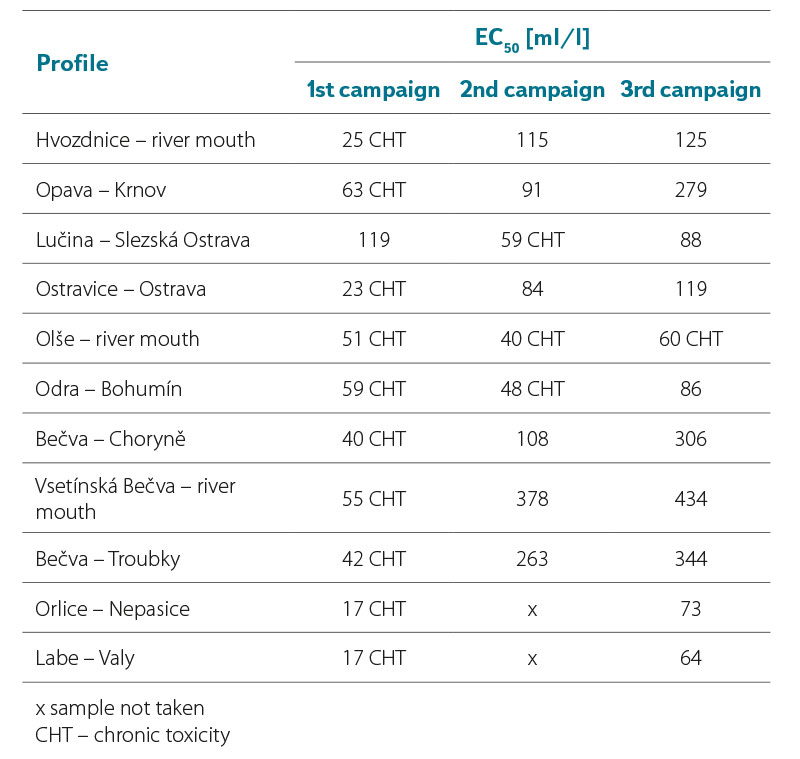
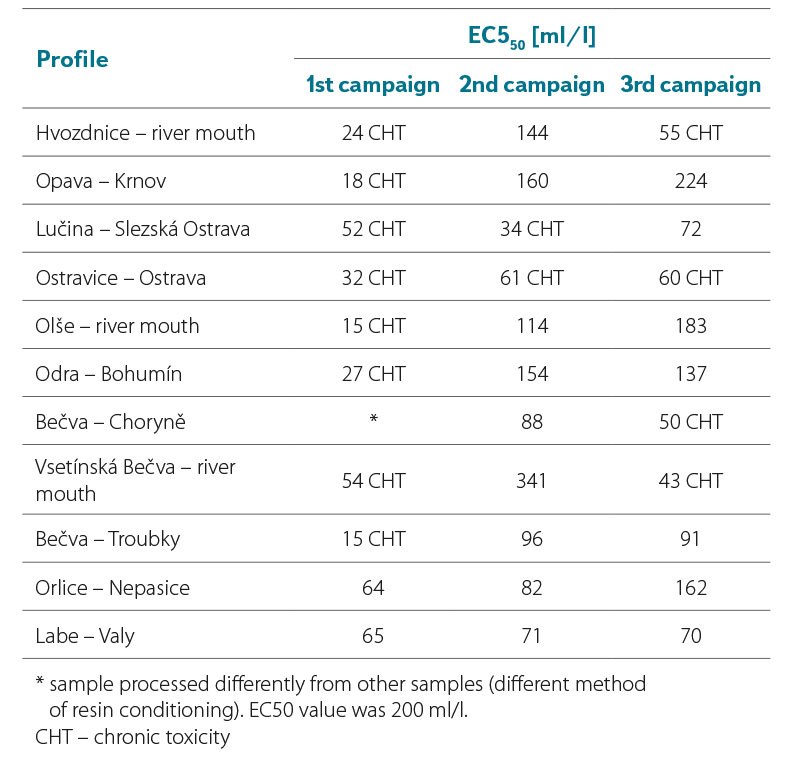
From the results in Tab. 3a and 3b, it can be seen that the lowest EC50 values were recorded in both years 2021 and 2022 during the first sampling campaign, where EC50 values were in a range of 15–119 ml/l. Samples collected in the second sampling campaign had EC50 values in a range of 40–378 ml/l, with values in 2021 being slightly higher than in 2022. In the third sampling campaign, the EC50 values ranged from 43 to 434 ml/l, while the values in 2021 were again slightly higher compared to 2022.
In general, we can say that almost all samples during the first sampling campaign had a greater effect on luminescence inhibition compared to samples from the second and third sampling campaigns. None of the EC50 values found was lower than 10 ml/l, i.e., the value indicating significant toxicity of the samples.
The EC50 values found for samples from some profiles in individual campaigns differed significantly. For example, the EC50 value of the sample from the Opava – Krnov profile in 2022 from the first campaign was among the lowest, with an EC50 value of 18 ml/l. In contrast, samples taken from this profile during the summer and autumn campaigns were among the least toxic. In contrast, the smallest difference in EC50 values was found for samples taken in 2022 on the Labe – Valy profile. Samples from this profile showed almost identical EC50 values in all three campaigns.
Tab. 3a. Results of the luminescent test with A. fischeri, EC50 values after 30 min in ml/l (2021)
Tab. 3b. Results of the luminescent test with A. fischeri, EC50 values after 30 min in ml/l (2022)
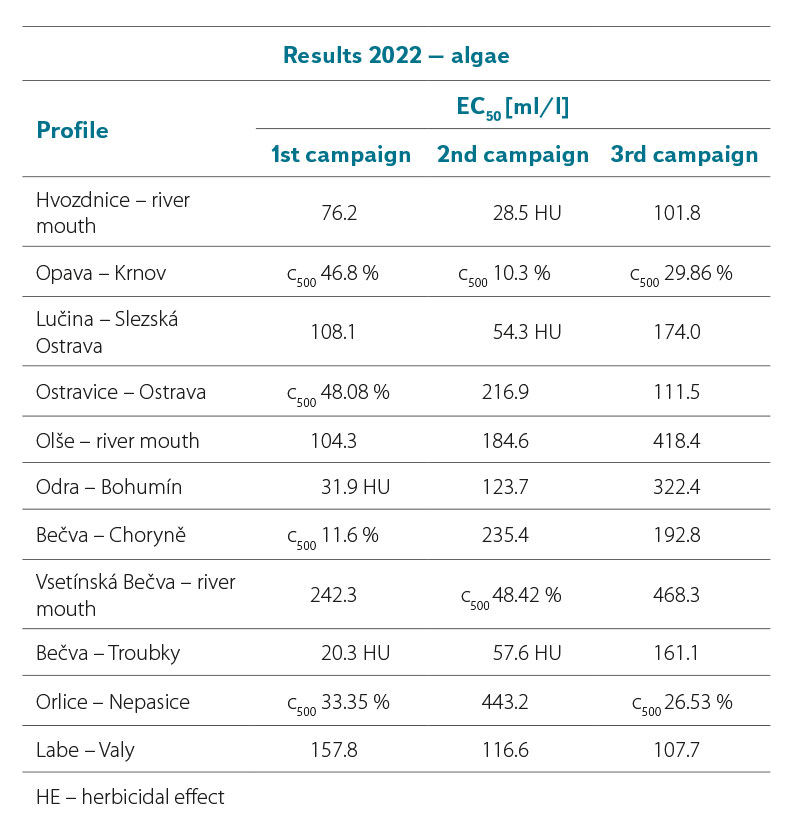
Toxicity test – miniaturized algae test with R. subcapitata
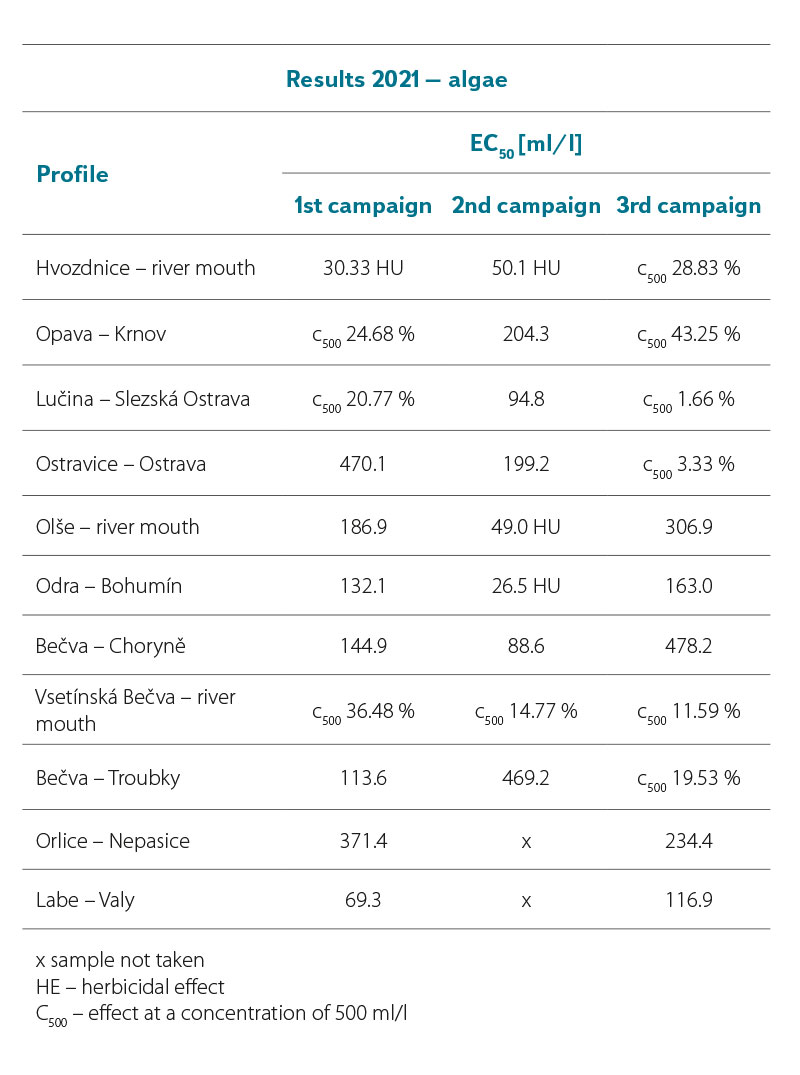
Tab. 4a and 4b show the results of the analysed surface water samples collected in 2021 and 2022. It is clear that a four-fold dilution (c 250 ml/l) of the samples in some cases caused 100 % inhibition of algae growth. The toxicity gradually decreased with further dilution. At a 100-fold dilution (i.e. a concentration of 10 ml/l) the samples had a toxic effect only exceptionally.
In the first sampling campaign of 2021, the sample in the Hvozdnice – river mouth profile showed increased toxicity; in the second sampling campaign, it was the sample in the Odra – Bohumín profile. In the third sampling campaign, no significant toxic effect of any of the samples was recorded. Similarly, in the first sampling campaign of 2022, only the sample from the Odra – Bohumín profile showed increased toxicity in the above-mentioned concentration. In the second sampling campaign, high toxicity was detected in this concentration in a sample from the Bečva – Troubky profile. In the third sampling campaign, no significant toxic effect of any of the samples was recorded. In the summer and autumn campaign, algae growth was stimulated in some of the examined samples due to the substances contained in them.
Although the algae inhibition effect appears to be significant in some concentrations, it should be noted that 1,000× concentrated surface water samples were analysed. These samples were subsequently diluted in the tests in order to find out which concentrations still have a significant inhibitory effect on algal growth and which, in contrast, have minimal effect.
Tab. 4a. Algae test results with R. subcapitata, EC50 in ml/l (2021)
Tab. 4b. Algae test results with R. subcapitata, EC50 in ml/l (2022)
Tab. 5. Results of Ames fluctuation test in the variant without metabolic activation S9-
Tab. 6. Comparison of Ames fluctuation test results in the variant without metabolic activation S9- and the variant with metabolic activation S9+
Genotoxicity test – Ames fluctuation test with metabolic activation (S9+) and without metabolic activation (S9-)
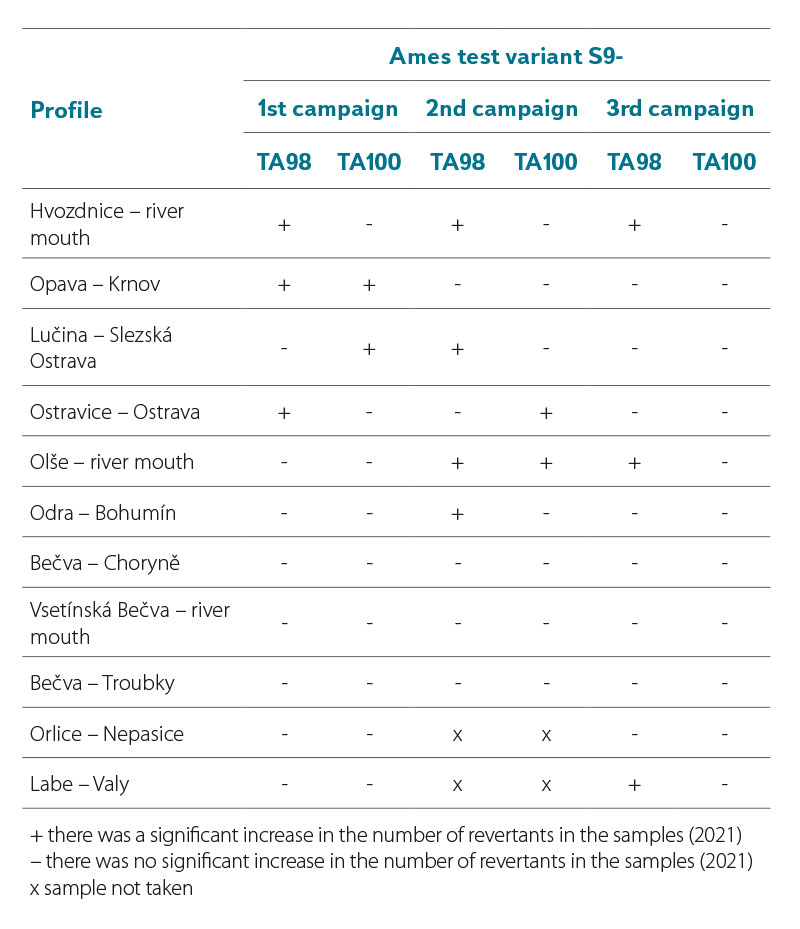
In 2021, the results of the Ames fluctuation test were obtained in the variant without metabolic activation of S9- (Tab. 5), where only direct mutagenic substances were monitored. In the first campaign, mutagenic substances were detected on the profiles of Hvozdnice – river mouth, Opava – Krnov, Lučina – Slezská Ostrava, and Ostravice – Ostrava. In the summer campaign, they were detected in the profiles Hvozdnice – river mouth, Lučina – Slezská Ostrava, Ostravice – Ostrava, Olše – river mouth, and Odra – Bohumín. In the autumn campaign, the Hvozdnice – river mouth, Olše – river mouth, and Labe – Valy profiles tested positive.
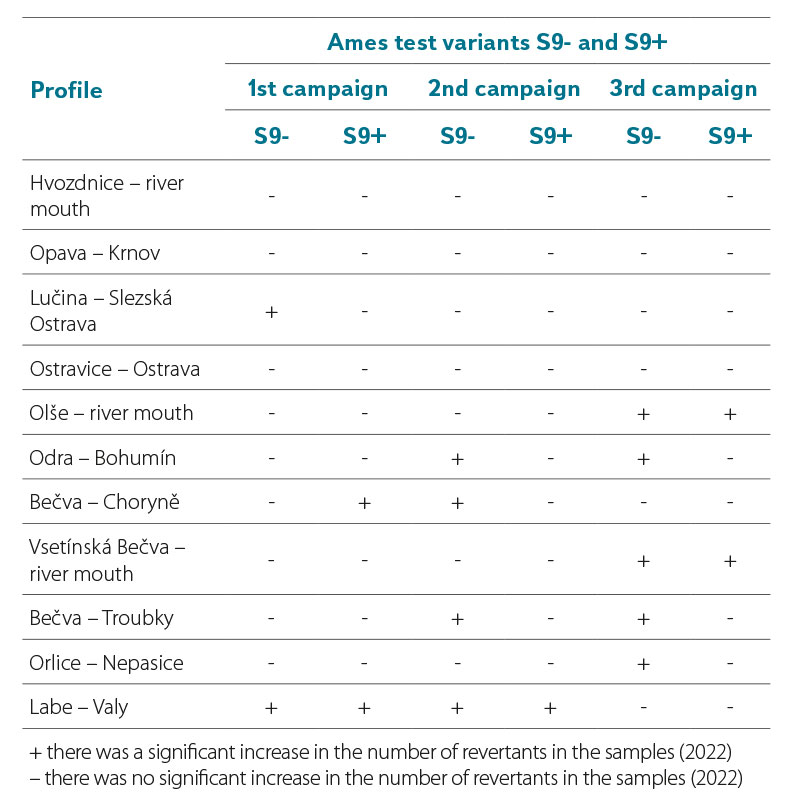
Tab. 6 shows a comparison of the result of the Ames fluctuation test in the variant without metabolic activation S9- and the variant with metabolic activation S9+ in surface water samples collected in 2022. If in the S9+ or S9- variant of the test, at least one of the Salmonella strains showed a significant increase in revertants compared to the control testing, the sample is marked as positive for the presence of mutagenic substances in the sample.
In the spring campaign, the presence of direct or indirect mutagenic substances was detected in a sample taken in the Lučina – Slezská Ostrava, Bečva – Choryně, and Labe – Valy profiles. The sample from the Labe was positive for the presence of mutagenic substances even in the second campaign. In this campaign, the presence of mutagenic substances was further detected in samples from the Odra – Bohumín, Bečva – Choryně, and Bečva – Troubky profiles. In the third campaign, samples from Olše, Odra, Bečva, and Orlice were positive. In 2022, direct mutagens were repeatedly detected in samples from the Labe – Valy, Odra – Bohumín, Bečva – Choryně, and Bečva – Troubky profiles.
Test to determine estrogen potential – Yeast estrogen test (YES test)
In the samples taken in 2021 and 2022, the YES test did not significantly record the induction ratio (IR) of β-galactosidase ≥ IR10 (where IR10 = 10 % (IRmax of the standard – IR of the negative control)) (Tab. 7a and 7b). The YES test did not show results indicating the presence of substances that would cause the expression of the reporter gene and the subsequent production of β-galactosidase to such an extent that the sample could be significantly marked as positive for the presence of estrogens or substances with estrogenic effects on the hER receptor. For six samples in 2021, the IR value was above 6 % of the maximum induction of β-galactosidase (Tab. 7a, highlighted). In Tab. 7b, two values in the third campaign (sample from the profiles Bečva – Troubky and Labe – Valy) are clearly marked, for which the IRmax value was above 9 %. If the value of these samples were ≥ 10 %, they would already be samples with a proven agonistic estrogenic effect. To verify the obtained results, the samples for which the maximum β-galactosidase induction value exceeded 6 % will be reanalysed by the YES test.
Tab. 7a. Results of estrogenic activity of samples in yeast estrogen tests (YES test) (2021)
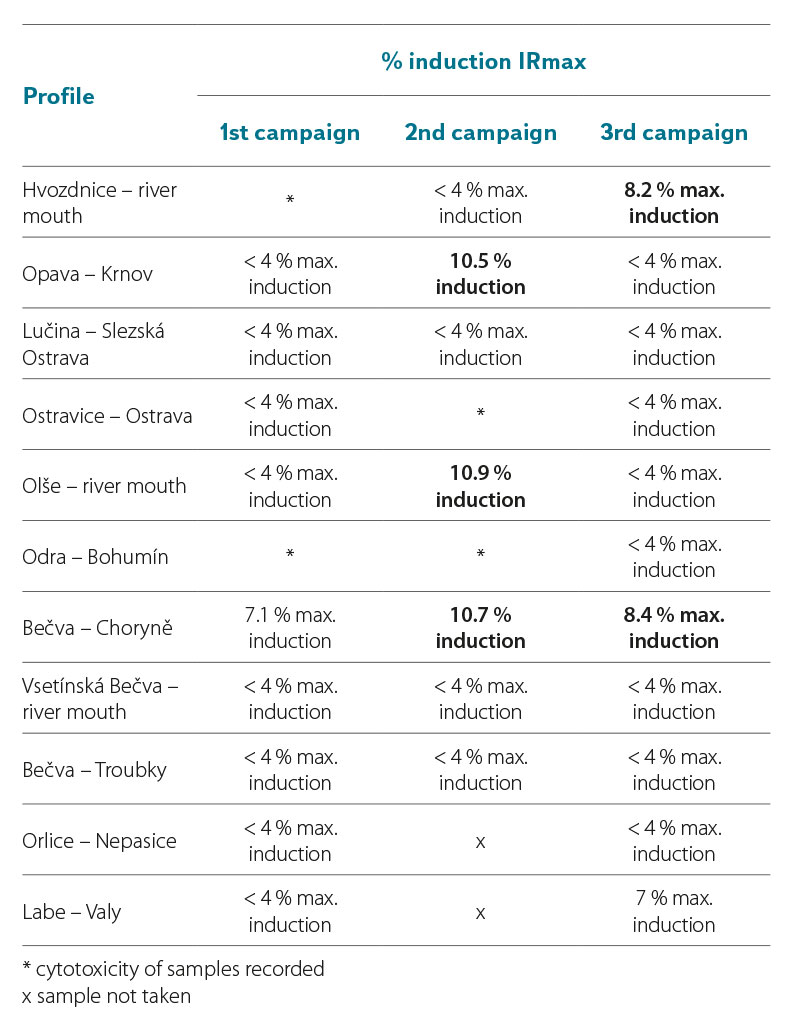
Tab. 7b. Results of estrogenic activity of samples in yeast estrogenity tests (YES test) (2022)
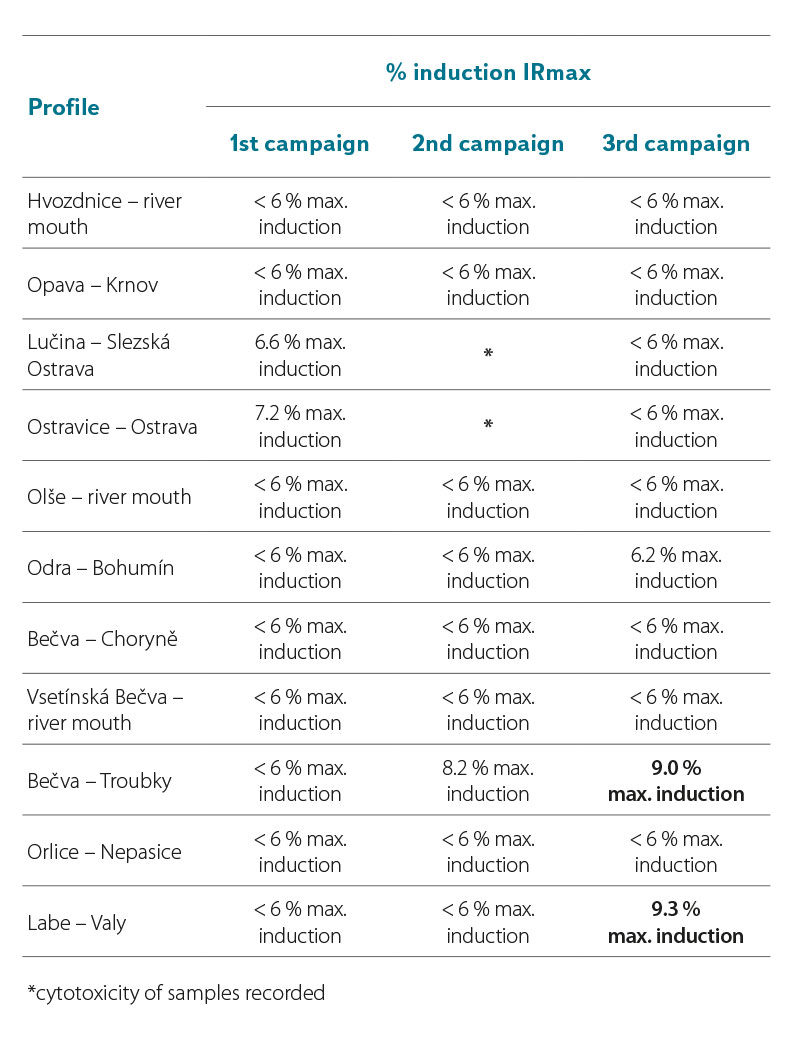
DISCUSSION AND CONCLUSION
Our measurements showed that even on the reference profiles, where the routine monitoring of selected parameters (carried out by Povodí state enterprises) showed long-term good water quality, chronic effects of pollution or their genotoxicity were determined in samples from some campaigns. This fact shows that routine monitoring apparently did not detect the substances or their mixtures that could cause the given effects, and the financial resources spent on its implementation therefore did not bring the required results for the assessment of the ecological water status, which is significantly influenced by the effect of pollution determined using EBM. In a situation where the list of priority and emergent pollutants to be monitored in waters is still growing, EBM has the potential to focus chemical monitoring on a purposeful basis, and thus to streamline its costs.
According to the Water Framework Directive, assessment of the chemical status and ecological status/potential of surface waters is carried out by monitoring priority substances and specific pollutants defined at the national level (in the Czech Republic according to Government Regulation 401/2015 Coll. and ČSN 75 7221 standard). However, these hazardous substances make up only a fraction of the total water toxicity. Chemical analysis is not able to cover all pollutants present in waters; therefore, the environmental impact of unregulated substances, as well as the effects of mixtures, is not considered.
Framework Directive 2000/60/EC requires Member States to set environmental objectives for each water body. If this goal is not met, the causes must be identified so that effective measures can be taken. For this purpose, it is necessary to have a suitable diagnostic system; however, such a tool is not often available. Most biological methods used according to the Framework Directive may not respond adequately to the presence of toxic substances and their mixtures in waters, or to other types of stressors. EBMs can provide overall information on the ecotoxicological effects of surface water pollution, and thus help in the evaluation of possible causes of unsatisfactory water status.
Based on the experience we have gained and the test results, it can be concluded that our project has demonstrated the applicability of the proposed EBM in routine practice and fully supports their introduction not only here, but also at the European level.
Acknowledgements
This article was supported by the Technological Agency of the Czech Republic project SS03010140 “Use of effect-based methods to assess the status of surface waters in the context of the Water Framework Directive”.
This article was translated on basis of Czech peer-reviewed original by Environmental Translation Ltd.