ABSTRACT
Aquatic organisms have a very good ability to reflect the conditions of the environment they live in and, therefore, they are often used to assess the ecological status of that particular environment. of juvenile fish assemblages (0+) represent an appropriate tool for monitoring the ecological status of watercourses as they show a very rapid response to changes in environmental conditions. The goal of this study was to assess assemblages of juvenile fish (0+) at 22 sites across the Czech Republic between 2019 and 2021. Electrofishing gear (battery – backpack unit) was used to sample the juvenile fish assemblages in shallow parts of rivers along riverbanks. The juvenile fish assemblages (0+) were relatively diverse; overall, a total of 36 species were found, with a minimum of four and a maximum of 15 species per site (average of nine species per site). Significant differences were observed in the assemblages of juvenile fish (0+) across the different sites and during the various years. The ecological status was assessed using the Czech multi-metric index (CZI). Significant differences in ecological status were observed – four sites showed a significant degradation of the ecological status, while four sites showed an improvement. The rest of the sites represented a stable ecological status (there was no significant improvement or degradation). Sites that showed the best ecological status, where juvenile fish assemblages corresponded to the natural character of rivers, were Orlice in Nepasice (Hradec Králové region) and Olše in Věřňovice (Moravian-Silesian region). In contrast, the lowest CZI values were detected at Ohře – Želina (Ústí nad Labem region), Dyje – Podhradí n. Dyjí, Dyje – Jevišovka (South Moravian region), and Cidlina – Sány (Central Bohemian region), suggesting a degraded ecological status. It was found that the degradation of the ecological status was caused neither by a significant change in suitable habitats for juvenile fish nor habitat loss, but instead generally by the presence of non-native species that significantly reduce the CZI value. Based on this research it can thus be concluded that significant changes in juvenile fish assemblages at a particular site can occur even within a very short time period (one year). Year-to-year changes in juvenile fish assemblages can be very significant, and for this reason it is important to perform monitoring every year.
INTRODUCTION
Many river systems are heavily altered or damaged by human activity [1], such as inappropriate hydromorphological modifications and manipulations at hydro-electric power stations [2, 3], introduction of invasive species, excessive input of nutrients, and pollution by hazardoussubstances [3]. These multi-stressors significantly affect entire aquatic ecosystems [1]. Water and its quality play an important role in terms of its usability as an irreplaceable raw material for countless sectors of human activity [4]. The same applies for the environment, to which a large number of organisms are bound in part or through their entire life cycle. The use of aquatic organisms (biota) as an indicator of ecological status has a justified significance [5]. Their physiological tolerance and ecological preferences are closely related to the environmental conditions in which they live, and they are able to quickly reflect environmental changes [6, 7]. Bioindicators are widely used to provide useful information about environmental changes or pollution and reflect long-term effects/stressors that do not act on organisms separately, but simultaneously [8]. Assessment methods are mostly based on the taxonomic composition of the community, which provides information on biological interactions, the internal formation of the community, as well as the functioning of the given ecosystem [9]. The assemblage of juvenile fish (i.e. 0+, where 0 means no experienced winter and + means an experienced vegetation season) therefore represents a suitable tool for monitoring the ecological status of watercourses, especially because most Bohemian and Moravian watercourses are stocked, i.e. subadult and adult fish are released [6]. Juvenile fish (0+) immediately reflect reproductive success or failure in the last spawning period and show a significantly faster response to changing environmental conditions than adult fish [6, 10]. In addition to the reproductive success of adult fish, the assemblage of juvenile fish (0+) is influenced by the survival of their early stages, which are very closely linked to the occurrence of suitable micro- to mesohabitats [11], such as shallow areas with sufficient food and shelter, so-called “fish nurseries” [10, 11]. The assemblage of juvenile fish (0+) is also shaped by seasonal and inter-seasonal changes in habitats as well as hydrological [12] and temperature regimes, which have a significant effect on the overall diversity and abundance of individual species [13, 14]. Environmental changes can be monitored through diversity on a local scale, based on species in a given assemblage (α diversity) or on a wider scale, between individual assemblages (β diversity, [15, 16]). The aim of this study was to assess the assemblage of juvenile fish (0+) and the ecological status of watercourses according to the Czech multi-metric index (CZI) within individual basins between 2019 and 2021 at 22 sites that represent closing profiles and important trunk streams in the Czech Republic.
METHODOLOGY
The biological assessment of the monitored watercourses was carried out using the natural fish assemblage, i.e., juvenile fish (0+). The methodology was compiled in such a way that it was possible to use it to carry out the catch, basic processing and assessment of fish samples (0+) [17, 18]. The chosen methodology represents the current status of the watercourses [19] where only fish that are a maximum of few months old are sampled. The ichthyological survey took place at 22 sites (Fig. 1), which were selected on the basis of previous findings from water quality monitoring carried out by the Czech Hydrometeorological Institute [19]. The monitored sites were located in the closing profiles and on the trunk streams of the Czech Republic (Fig. 1). Sampling sites for catching juvenile fish (0+) were located below municipalities and adjacent agglomerations due to possible influence by technical modifications, weir manipulations, discharge of waste water, and surface sources of pollution, especially in important agricultural areas. Thanks to the given sampling design, it was possible to objectively determine the influence of human activity on the assemblage of juvenile fish (0+) between individual basins, as well as across the Czech Republic.
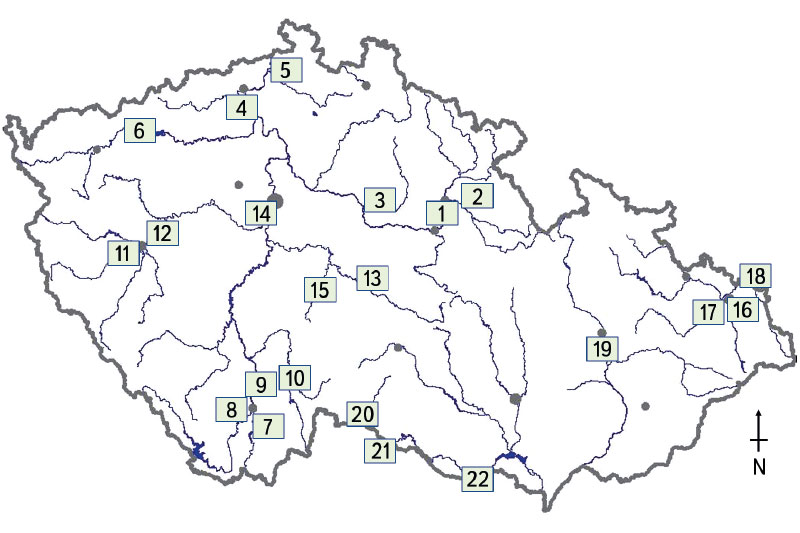
Fig. 1. Monitored watercourses with marked profiles where juvenile fish (0+) were caught: 1) Labe – Hradec Králové, 2) Orlice – Nepasice, 3) Cidlina – Sány, 4) Labe – Litoměřice, 5) Ploučnice – Děčín (Březiny), 6) Ohře – Želina, 7) Malše – Roudné, 8) Vltava – Boršov, 9) Vltava – Hluboká nad Vltavou, 10) Lužnice – Veselí nad Lužnicí, 11) Mže – Plzeň, 12) Berounka – Plzeň, 13) Sázava – Zruč nad Sázavou, 14) Vltava – Praha (Vrané), 15) Želivka – Poříčí, 16) Ostravice – Ostrava, 17) Odra – Ostrava (Svinov), 18) Olše – Věřňovice, 19) Morava – Blatec, 20) Moravská Dyje – Písečné, 21) Dyje – Podhradí, 22) Dyje – Jevišovka
Catching the fish
Fish catches (0+) were carried out from the second half of August to the second half of September. Late summer is a suitable period to sample juvenile fish (0+) due to relatively low and stable flows. The abundance of juvenile fish (0+) is already relatively stable compared to the high mortality that occurs during the first weeks to months after hatching [10]. During this period, juvenile fish (0+) still stay in the shallow sections along the banks and do not yet move to the deeper parts of the watercourses (to the wintering grounds), which usually happens during the autumn months [10]. In this period, juvenile fish (0+) are already sufficiently mature, their identification features are similar to adults, and their identification can be carried out directly in the field [17, 20].
Catching the fish was carried out along the banks of a watercourse (Fig. 2) with a battery-powered electric unit (type SEN and LENA from the Bednář company) with an output frequency of 50–95 Hz [10, 21]. The fish were caught using a direct pulsed current, which is not dangerous for the fish’s health in the given frequency range [17, 20]. The length of the fished section depended on the amount of mesohabitats (shallow stream sections, dead wood, aquatic and flooded terrestrial vegetation, standing water) and ranged from 50 m to 200 m (median 100 m). The monitored section was divided into several sub-sections in order to capture a significant part of the environmental variability and the total assemblage of juvenile fish (0+). Following the catch, the fish were identified directly at a given site (Fig. 3).

Fig 2. Juvenile fish assamblages sampling in shallow sections along the riverbank
Fig. 3. The determination of juvenile fish
DATA PROCESSING
The ecological status assessment of the monitored watercourses was carried out using the Czech multi-metric index (CZI), which combines several metrics, whose results are combined into a multi-metric output and include several attributes of the assemblage. Metrics that describe and assess environmental conditions include altitude, watercourse order according to Strahler, sea-drainage area, watercourse type (A – mountain streams to G – lowland rivers), and typical taxa for a given type of watercourse, as well as non-native species, which significantly reduce the resulting index value [16]. The multi-metric index was calculated according to the following equation:
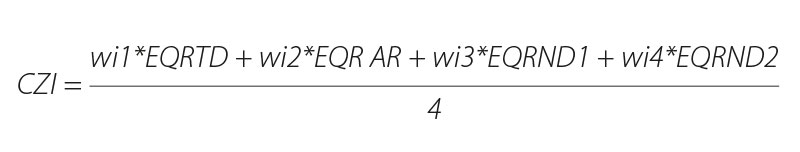
where
wi is the weight of the metric when calculating CPI
TD number of typical taxa
AR abundance of rheophiles (current-loving species)
ND1 presence of undesirable species
ND2 – relative representation of undesirable species – takes on values from 0 to 1 (category CZI, 0–0.2 destroyed; > 0.2–0.4 damaged; > 0.4–0.6 medium; > 0.6–0.8 good and > 0.8–1 excellent). The upper and lower limits of the metric values are used to calculate the Ecological Quality Ratio (EQR), i.e., the ratio between the detected and expected (reference) values [16].
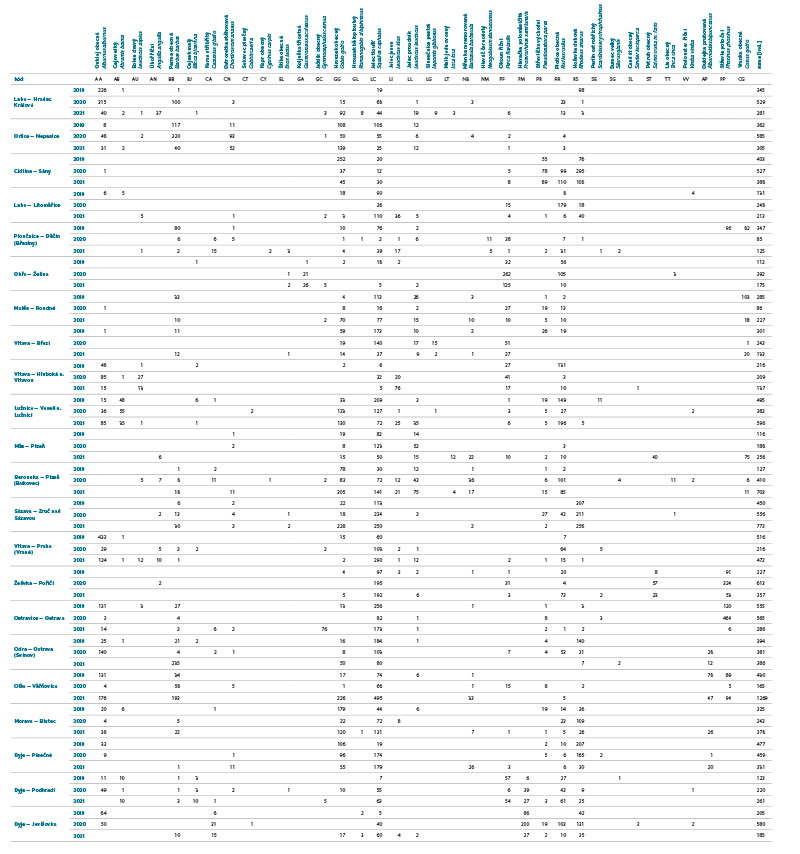
Differences in the juvenile fish assemblage were assessed in the R software program ver. 4.2.2 [21] through the PERMANOVA (Permutational Multivariate Analysis of Variance) method and displayed using multiple scaling – NMDS (Non-Metric Multidimensional Scaling). Visualization of the differences in the fish assemblage was shown through the code designation of individual species (AA – Alburnus alburnus, AB – Abramis brama, AN – Anguilla anguilla, AP – Alburnoides bipunctatus, AU – Leuciscus aspius, BB – Barbus barbus, BJ – Blicca bjoerkna, CA – Carassius gibelio, CG – Cottus gobio, CN – Chondrostoma nasus, CT – Cobitis taenia, CY – Cyprinus carpio, EL – Esox lucius, GA – Gasterosteus aculeatus, GC – Gymnocephalus cernua, GG – Gobio gobio, GL – Romanogobio albipinnatus, LC – Squalius cephalus, LG – Lepomis gibbosus, LI – Leuciscus idus, LL – Leuciscus Leuciscus, LT – Lota lota, NB – Barbatula barbatula, NM – Neogobius melanostomus, PF – Perca fluviatilis, PM – Proterorhinus semilunaris, PP – Phoxinus phoxinus, PR – Pseudorasbora parva, RR – Rutilus rutilus, RS – Rhodeus amarus, SE – Scardinius erythrophthalmus, SG – Silurus glanis, SL – Sander lucioperca, ST – Salmo trutta m. fario, TT – Tinca tinca, VV – Vimba vimba). Comparison of differences in the juvenile fish assemblage between individual years (2019–2021) was performed using the Euclidean distance (Jaccard index). The Cao index [22] was used to assess (beta) diversity of the assemblage between individual sites in the monitored period.
RESULTS
The assemblage of juvenile fish was relatively rich, with 36 species recorded at 22 sites. There were significant differences in the composition of the species community between individual sites; at least four species were recorded per site (Cidlina – Sány in 2019); the most species (15) were caught in 2021 at the Labe – Hradec Králové site (the section was fished below the weir near the village of Vysoká nad Labem). In the monitored period, an average of 9 species were caught at the sites (an average of 7.1 species per site was recorded in 2019, 8.7 in 2020, and 9.7 in 2021). Among the species with the highest abundance were European chub (Squalius cephalus ∑6156 ind. [individuum], Fig. 4, Tab. 1), gudgeon (Gobio gobio ∑2976 ind., Fig. 4, Tab. 1), European bitterling (Rhodeus amarus ∑2518 ind., Fig. 4, Tab. 1), common bleak (Alburnus alburnus ∑2447 ind., Fig. 4, Tab. 1), common roach (Rutilus rutilus ∑2007 ind., Fig. 4, Tab. 1), and barbel (Barbus barbus ∑1434 ind., Fig. 4, Tab. 1). The Catch Per Unit Effort (CPUE) fluctuated significantly between individual years and sites; the minimum value o CPUE was recorded in 2019 at the Berounka in Pilsen (Bukovec) – 0.3 ind.m-1 (Fig. 5), and the maximum was 25.4 ind.m-1 at Olše in Věřňovice in 2021 (Fig. 5).
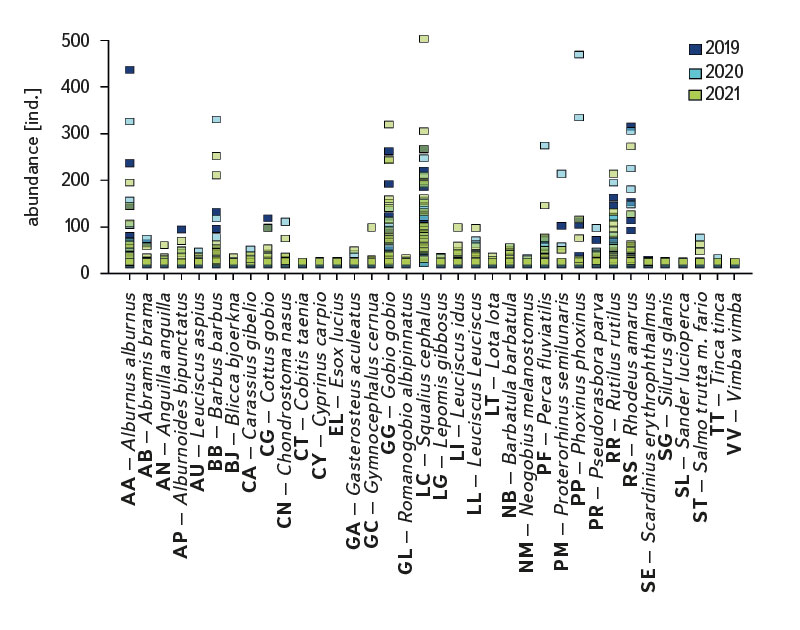
Fig. 4. The results of juvenile fish survey, abundance of fish species between 2019–2021
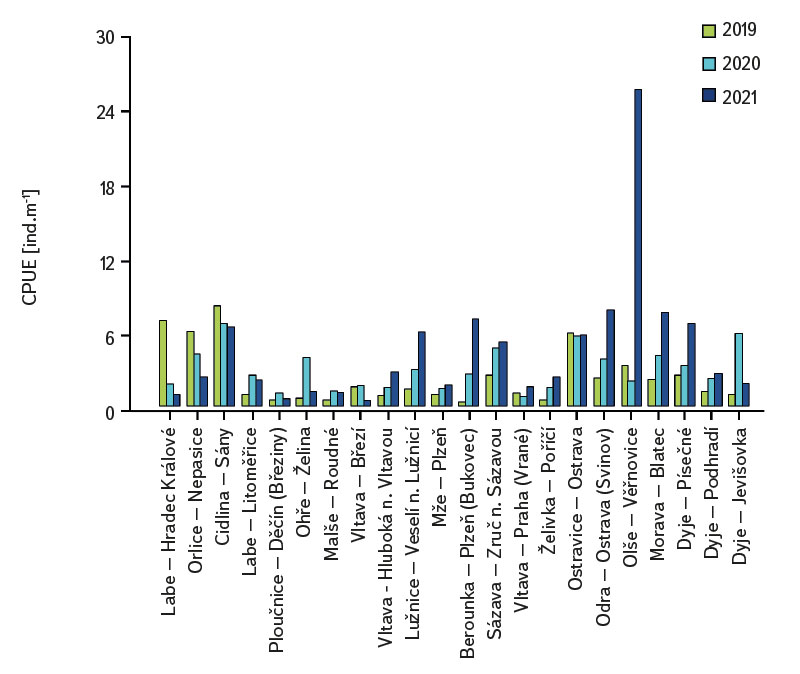
Fig. 5. The results of catch per unit effort (CPUE) at monitored localities between 2019–2021
Tab 1. Summary of juvenile fish (0+) caught at monitored sites for the period 2019–2021
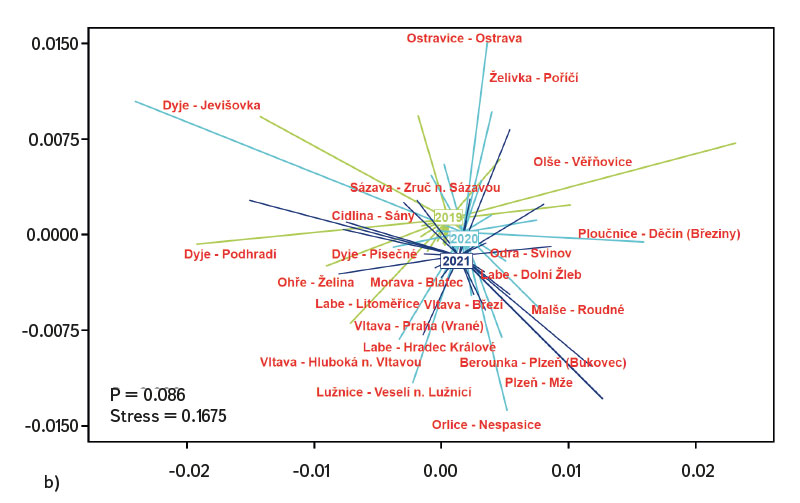
The average value of CPUE between sites and years was 3.3 ind.m-1. A medium and higher CPUE value was recorded in 23 cases during the monitored period (in 2019 at five sites, and in 2020 and 2021 at nine sites). Similar to CPUE, fish biomass showed high variability between years and sites. The lowest values of 0.4 g.m-1 were recorded in 2019 on the Vltava in Prague (Vrané, Fig. 6), while the highest values of 5.0 g.m-1 were recorded in 2021 on the Odra in Ostrava (Svinov, Fig. 6). The average value of biomass between sites in the monitored period reached 1.8 g.m-1. The medium and higher value of biomass was recorded in 27 cases (in 2019 it was found at seven sites, in 2020 at eight sites, and in 2021 at 12 sites). The ecological status assessment according to the Czech multi-metric index (CZI) showed significant changes in the monitored sites that occurred during 2019–2021. Degradation of the status was recorded at four monitored sites compared to previous years (Labe – Hradec Králové, Ploučnice – Děčín/Březiny, Mže – Plzeň, Dyje – Podhradí, Fig. 7). The lowest CZI values, and thus the worst ecological status (i.e., destroyed and damaged), were recorded at the following sites: Ohře – Želina (0.200, Fig. 7), Dyje – Jevišovka (0.295, Fig. 7), Cidlina – Sány (0.305, Fig. 7), and Dyje – Podhradí (0.344, Fig. 7). At the Ohře in Želina, the population was mainly dominated by European perch (Perca fluviatilis), with a minor proportion of common roach and three-spined stickleback (Gasterosteus aculeatus). In the community on the Dyje in Jevišovka, the majority consisted of western tubenose goby (Proterorhinus semilunaris), European bitterling, and common roach. On the Cidlina in Sány, the majority share of the community was formed by European bitterling, gudgeon, common roach, and topmouth gudgeon (Pseudorasbora parva). On the Dyje in Podhradí, European chub, common roach, and European perch dominated the fish community. An improvement in ecological status was detected in a total of nine sites (Fig. 7). The most significant improvement during the monitored period was recorded at four sites, i.e., on the Labe in Litoměřice, Lužnice in Veselí nad Lužnicí, Vltava in Prague (Vrané), and Dyje in Písečné. At the sites of the Orlice in Nepasice and the Olše in Věřňovice, the ecological status reached first class (i.e., excellent). At the remaining sites, the situation was rather stable – there was neither significant improvement nor degradation (Fig. 7). Multivariate analyses showed significant differences in the juvenile fish assemblage in 2019–2021 (P = 0.011, Fig. 8a), but no differences in community diversity across the monitored sites were proven (P = 0.086, Fig. 8b).
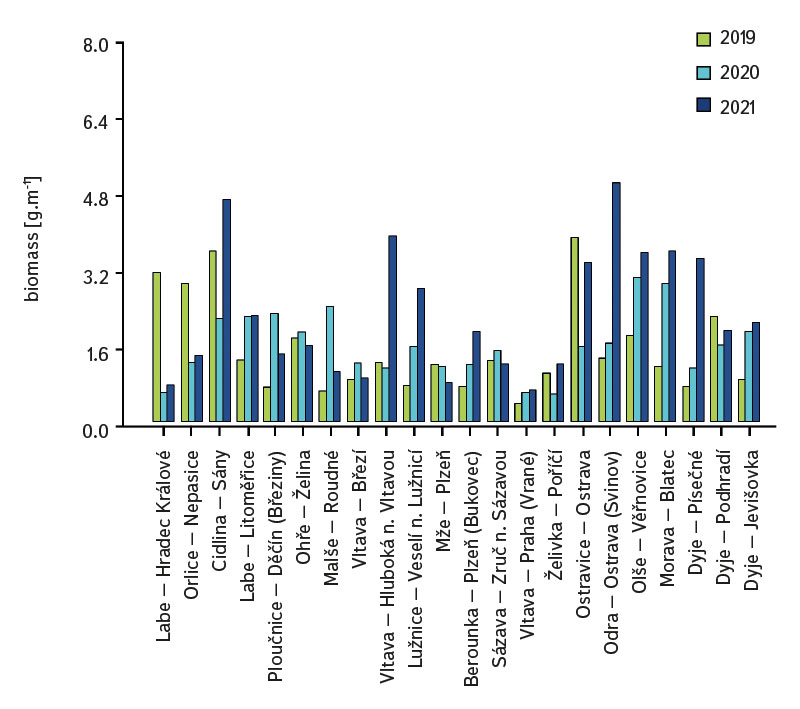
Fig. 6. Biomass of juvenile fish at monitored localities between 2019–2021
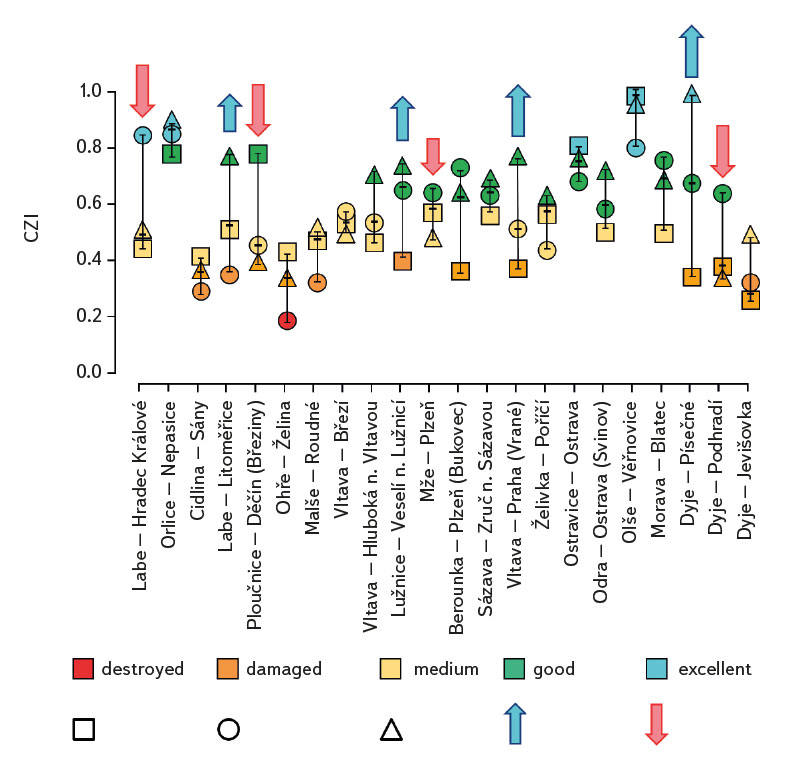
Fig. 7. The evaluation of ecological status using the Czech multimetric index (CZI) at monitored localities between 2019–2021
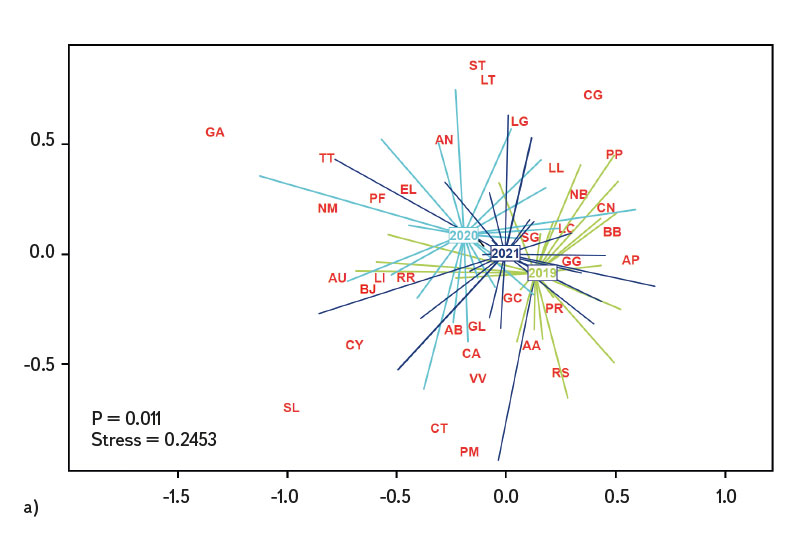
 Fig. 8. Similarities between monitored localities based on juvenile fish assemblages, a) the results of nonmetric multidimensional scaling of juvenile fish assemblages between 2019–2021, b) differences in juvenile fish assemblages between localities and across years 2019–2021
Fig. 8. Similarities between monitored localities based on juvenile fish assemblages, a) the results of nonmetric multidimensional scaling of juvenile fish assemblages between 2019–2021, b) differences in juvenile fish assemblages between localities and across years 2019–2021

Catch of juvenile fish in a technically heavily modified river basin

The Ohře river near the Želinský meander

Selection of an appropriate flow section depending on the variability of the environment


After determination, the fish were gently released back into the river
DISCUSSION
The study was conducted across the Czech Republic. Individual watercourses and sites differed significantly not only in terms of water bearing, geomorphology, but also in the technical modifications of the riverbed. At all 22 monitored sites, the assemblage of juvenile fish was very diverse. Species diversity varied across individual years and sites; a total of 36 species were recorded (a minimum of four and a maximum of 15 species per site). CPUE values showed a relatively high variability between sites and monitored years (Fig. 5). The lowest CPUE values (0.3 ind.m-1, Fig. 5) were recorded on the Berounka in Pilsen in 2019; however, in 2021, CPUE values of 6.8 ind.m-1 were recorded (Fig. 5). The highest CPUE values were 25.4 ind.m-1 in Olše in Věřňovice in 2021; however, significantly lower abundances were recorded in previous years (4.5 and 2.1 ind.m-1, Fig. 5). Similarly, the biomass also showed great variability in the monitored period between sites and years; the lowest values were found on the Vltava in Prague (0.4 g.m-1, Fig. 6) in 2019, but in 2021 the biomass reached almost double the values (Fig. 6). The highest values of 5.0 g.m-1 were in 2021 on the Odra in Ostrava (the Svinov district, Fig. 6); however, more than three times lower biomass values were recorded in previous years (Fig. 6). Significant differences in biomass and CPUE between individual years within the same site may be related to interannual differences, temperature fluctuations or water level fluctuations (floods, drought), which have a significant effect on the reproductive potential of fish and their entire community [6, 24, 25]. Differences in both abundance and biomass can also be influenced by interannual biological cycles, such as the sizes of individual cohorts entering breeding [6, 10], which can vary significantly between individual years. They can also be caused by the fluctuation of available food, i.e., a change in the community of micro and macrozoobenthos, which represents an important source of food for juvenile fish [24, 26, 27]. Among other things, even significant temperature fluctuations have a noticeable effect on fish reproduction [13, 28], because higher water temperatures can contribute to an earlier spawning time, while a sudden drop can slow down or delay fish spawning [13, 29]. It can be assumed that a significant drop in temperature in the spring season can also cause the absence of a cohort, especially in fish with batch spawning, such as European chub and common nase. In 2020, February and March were significantly above average in temperature, while May was very cold (with a deviation of -2.1 °C from normal, [31]). In a number of sites, a missing cohort was recorded in a number of sites this year during fish catches, or the size spectrum ranged only in two categories (about 20–30 mm and 40–50 mm of body length), and the middle category of 30–40 mm was almost absent (this mainly concerned European chub and common nase). Despite significant differences in the abundance of individual species (Fig. 4) and significant variability in species diversity (Fig. 8a), and due to significant differences between a number of sites (Fig. 8b), no statistically significant differences were found in the assemblage of juvenile fish between the monitored sites (P = 0.086, Fig. 8b); however, this value is quite close to the significance level (P = 0.05). In contract, significant differences in the assemblage were recorded in 2019–2021 (P = 0.011, Fig. 8a), when the species variability changed noticeably during the monitored years (Fig. 8a). Inconclusive differences in the assemblage of juvenile fish between sites could be caused by a significant representation of eurytopic species, as the monitored sites are more probably to be found in the lower parts of watercourses, and therefore the communities between sites could be quite similar. In contrast, significant differences in the fish community between monitored years may point to fundamental changes that take place during individual years, or reflections of normal interannual fluctuations of an otherwise stable community may have been captured [25]. According to the Czech multi-metric index, two sites almost consistently showed the best composition of the juvenile fish assemblages, i.e. excellent ecological status (0.863–1.0 CZI, Fig. 7). These were Orlice in Nepasice and Olše in Věřňovice. The banks and riverbed were made of medium coarse gravel to sand. There was a considerable amount of mesohabitats that were suitable both for reproduction and for the growth and survival of the spawning community [32], i.e., river shallows with a low current speed and a significant amount of dead wood, which formed a suitable habitat with enough food and shelter [10, 33]. In contrast, the lowest CZI values (0.200, 0.296, 0.305, 0.344, Fig. 7), which represent the “worst” state (destroyed to damaged), were found on the Ohře in Želina, the Dyje in Jevišovka, the Cidlina in Sány and the Dyje in Podhradí. The Ohře and Dyje were influenced by the adjacent water reservoirs (Nechranická, Vranovská, and Novomlýnská reservoirs), into which they form the main tributaries. Simultaneously, the reservoirs also influence the resulting assemblage of juvenile fish (e.g., by the height of the swelling and reproduction of part of the reservoir stock in tributaries). In the monitored sections, the riverbed was relatively shallow, stony to sandy and only in places overgrown with algal growths and aquatic macrophytes. On the Ohře, the species community was relatively poor, with the predominance of European perch in particular, with a smaller occurrence of common roach and three-spined stickleback. In the spring, part of the stock travels from the dam to the tributaries, where it reproduces [34, 35]. In the early spring months, perch [35] and then roach [36] reproduce. European perch is able to actively hunt smaller juvenile fish at a size of 25–30 mm. It normally grows to this size during July and August [37–39]. Its great abundance, together with its enormous predatory potential, allows it to prevail in the assemblage of juvenile fish, where it subsequently forms a dominant share. The low values of the Czech multi-metric index on the Dyje in Podhradí and Jevišovka were caused by the relatively low abundance of rheophilic species, higher abundance of eurytopic species such as common roach and European bitterling, and especially the presence of non-native species such as western tubenose goby, topmouth gudgeon, and Prussian carp (Carassius gibelio). On the Cidlina in Sány, the abundance of gudgeon decreased in the fish community in the given period, and common roach and topmouth gudgeon gradually began to dominate. The community was influenced by the proximity of the Žehuňský pond, which had an effect on the flow conditions and temperature regime and can also serve as a reservoir for non-native species, such as topmouth gudgeon. According to the CZI, the degradation of the ecological status during the monitored three-year period was recorded at four sites (Labe – Hradec Králové, Ploučnice – Děčín/Březiny, Mže – Plzeň, and Dyje – Podhradí, Fig. 4). In the given period, no significant change of mesohabitats was recorded at the monitored sites (e.g., technical modifications of the riverbed or excessive overgrowth of the riverbed with macrophytes due to low flows). The deterioration was mainly caused by the presence of non-native species, which significantly reduce the value of the CZI. These species already expand further from newly colonized areas or are intentionally or unintentionally expanded with fish stocks [40, 41], or escape from ponds and other water bodies (fish production, ornamental ponds and lakes), which are situated in the upper parts of the basin [42]. In contrast, an improvement in the status during 2019–2021 was recorded at four sites (Vltava – Hluboká nad Vltavou, Vltava – Vrané nad Vltavou, Želivka – Poříčí, Dyje – Písečné, Fig. 4). The improvement may be related to the creation of suitable mesohabitats for fingerling survival, which arose as a result of more significant hydrological events (i.e., increased water levels), which were recorded mainly in the spring and autumn months of 2020 (Czech Hydrometeorological Institute, unpublished data). Significant fluctuations in water levels can result in hydromorphological changes in riverbeds [43, 44], especially cleaning of the riverbeds from fine inorganic and organic material (detritus), which can contribute to the creation of a number of mesohabitats [11]. These can subsequently be used for individual stages of juvenile fish (0+) [32, 45, 46].
CONCLUSION
The study results point to the fact that the assemblage of juvenile fish (0+) represents a suitable indicator of the ecological status of our watercourses and is directly and indirectly influenced by the natural conditions in a given year. The improvement of the ecological status in many sites was probably caused primarily by increased water levels, which act as an important channel-forming element and which caused the removal of sediments and the creation of suitable mesohabitats for the reproduction and subsequent survival of the first stages of juvenile fish (0+), especially in rheophilic species. However, degradation of the ecological status was not caused by a significant change in suitable habitats or their sudden decline, but mainly by the presence of non-native species, which significantly reduce the CZI index value. The conclusions of our survey point to the fact that significant changes in the assemblage of juvenile fish can occur at the same site even in a very short period of time (one year). Interannual changes can be very significant, so it is important to carry out monitoring every year in order to be able to separate “normal” fluctuations from fundamental changes taking place in the assemblage of juvenile fish (0+).
The Czech version of this article was peer-reviewed, the English version was translated from the Czech original by Environmental Translation Ltd.